Abstract
Purpose
To study the contribution of embryo chromosomal abnormalities in primary and secondary recurrent pregnancy loss (RPL) and to analyze the recurrence of chromosomal constitution in miscarriages from the same couple.
Methods
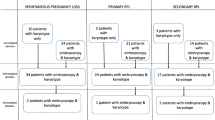
Retrospective study of abortion karyotypes in RPL families based on the mother’s primary or secondary RPL status (563 embryo specimens, 335 samples from primary, and 228 samples from secondary RPL). RPL was defined as two or more consecutive miscarriages. One hundred eight cases of recurrent embryo/fetal loss in 51 families were analyzed to assess the probability of having the same karyotype pattern (recurrent normal or recurrent abnormal) in both previous and subsequent pregnancy loss. The karyotypes of abortions were established using standard cytogenetic analysis, as well as interphase fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH).
Results
The frequency of aberrations was 43.9% in abortions from primary RPL versus 52.6% in secondary RPL (p = 0.041). Women 35 years of age or older were the main contributors to this difference. The odds ratio of a subsequent abortion having the same karyotype pattern (normal or abnormal) as the previous one was 6.98 (p = 0.0013).
Conclusion
The frequency of abnormalities is higher in abortions from the secondary RPL versus primary RPL group, and this difference is due to the relative deficiency of miscarriages with abnormal karyotypes in older women with primary RPL. The probability of having the same karyotype pattern (recurrent normal or recurrent abnormal) in the previous and subsequent abortion is increased significantly compared with chance.


Similar content being viewed by others
References
Kolte AM, Nielsen HS, Moltke I, Degn B, Pedersen B, Sunde L, et al. A genome-wide scan in affected sibling pairs with idiopathic recurrent miscarriage suggests genetic linkage. Mol Hum Reprod. 2011;17:379–85.
Miskovic S, Culic V, Konjevoda P, Pavelic J. Positive reproductive family history for spontaneous abortion: predictor for recurrent miscarriage in young couples. Eur J Obstet Gynecol Reprod Biol. 2012;161:182–6.
Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73:300–4.
Sullivan AE, Silver RM, LaCoursiere DY, Porter TF, Branch DW. Recurrent fetal aneuploidy and recurrent miscarriage. Obstet Gynecol. 2004;104:784–8.
Nikitina TV, Sazhenova EA, Tolmacheva EN, Sukhanova NN, Kashevarova AA, Skryabin NA, et al. Comparative cytogenetic analysis of spontaneous abortions in recurrent and sporadic pregnancy losses. Biomed Hub. 2016;1:1–11.
Ozawa N, Ogawa K, Sasaki A, Mitsui M, Wada S, Sago H. Maternal age, history of miscarriage, and embryonic/fetal size are associated with cytogenetic results of spontaneous early miscarriages. J Assist Reprod Genet. 2019;36:749–57.
Bianco K, Caughey AB, Shaffer BL, Davis R, Norton ME. History of miscarriage and increased incidence of fetal aneuploidy in subsequent pregnancy. Obstet Gynecol. 2006;107:1098–102.
Rubio C, Buendia P, Rodrigo L, Mercader A, Mateu E, Peinado V, et al. Prognostic factors for preimplantation genetic screening in repeated pregnancy loss. Reprod BioMed Online. 2009;18:687–93.
Hodes-Wertz B, Grifo J, Ghadir S, Kaplan B, Laskin CA, Glassner M, et al. Idiopathic recurrent miscarriage is caused mostly by aneuploid embryos. Fertil Steril. 2012;98:675–80.
Kort JD, McCoy RC, Demko Z, Lathi RB. Are blastocyst aneuploidy rates different between fertile and infertile populations? J Assist Reprod Genet. 2018;35:403–8.
Alberman E. The epidemiology of repeated abortion. In: Beard RW, Sharp F, editors. Early pregnancy loss: mechanisms and treatment. London: Springer-Verlag; 1988. p. 9–17.
Brigham SA, Conlon C, Farquharson RG. A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage. Hum Reprod. 1999;14:2868–71.
Shapira E, Ratzon R, Shoham-Vardi I, Serjienko R, Mazor M, Bashiri A. Primary vs. secondary recurrent pregnancy loss--epidemiological characteristics, etiology, and next pregnancy outcome. J Perinat Med. 2012;40:389–96.
Egerup P, Kolte AM, Larsen EC, Krog M, Nielsen HS, Christiansen OB. Recurrent pregnancy loss: what is the impact of consecutive versus non-consecutive losses? Hum Reprod. 2016;31:2428–34.
Ostroverkhova NV, Nazarenko SA, Lebedev IN, Cheremnykh AD, Nikitina TV, Sukhanova NN. Detection of aneuploidy in spontaneous abortions using the comparative hybridization method. Genetika. 2002;38:1690–8.
Lebedev IN, Ostroverkhova NV, Nikitina TV, Sukhanova NN, Nazarenko SA. Features of chromosomal abnormalities in spontaneous abortion cell culture failures detected by interphase FISH analysis. Eur J Hum Genet. 2004;12:513–20.
Bell KA, van Deerlin PG, Haddad BR, Feinberg RF. Cytogenetic diagnosis of “normal 46,XX” karyotypes in spontaneous abortions frequently may be misleading. Fertil Steril. 1999;71:334–41.
Jarrett KL, Michaelis RC, Phelan MC, Vincent VA, Best RG. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46,XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198–203.
Nikitina TV, Lebedev IN, Sukhanova NN, Sazhenova EA, Nazarenko SA. A mathematical model for evaluation of maternal cell contamination in cultured cells from spontaneous abortions: significance for cytogenetic analysis of prenatal selection factors. Fertil Steril. 2005;83:964–72.
Carp H, Toder V, Aviram A, Daniely M, Mashiach S, Barkai G. Karyotype of the abortus in recurrent miscarriage. Fertil Steril. 2001;75:678–82.
Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27:2297–303.
Feichtinger M, Wallner E, Hartmann B, Reiner A, Philipp T. Transcervical embryoscopic and cytogenetic findings reveal distinctive differences in primary and secondary recurrent pregnancy loss. Fertil Steril. 2017;107:144–9.
Massalska D, Bijok J, Kucinska-Chahwan A. Chromosomal abnormalities in the first-trimester spontaneous pregnancy loss. Austin J Obstet Gynecol. 2018;5:1116.
Demko ZP, Simon AL, McCoy RC, Petrov DA, Rabinowitz M. Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism-based preimplantation genetic screening. Fertil Steril. 2016;105:1307–13.
Lenzi ML, Smith J, Snowden T, Kim M, Fishel R, Poulos BK, et al. Extreme heterogeneity in the molecular events leading to the establishment of chiasmata during meiosis I in human oocytes. Am J Hum Genet. 2005;76:112–27.
Menasha J, Levy B, Hirschhorn K, Kardon NB. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: new insights from a 12-year study. Genet Med. 2005;7:251–63.
Subramaniyam S, Pulijaal VR, Mathew S. Double and multiple chromosomal aneuploidies in spontaneous abortions: a single institutional experience. J Hum Reprod Sci. 2014;7:262–8.
Kurahashi H, Tsutsumi M, Nishiyama S, Kogo H, Inagaki H, Ohye T. Molecular basis of maternal age-related increase in oocyte aneuploidy. Congenit Anom (Kyoto). 2012;52:8–15.
Coulam C. What about superfertility, decidualization, and natural selection? J Assist Reprod Genet. 2016;33:577–80.
van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta. 1822;2012:1951–9.
Sahoo T, Dzidic N, Strecker MN, Commander S, Travis MK, Doherty C, et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet Med. 2017;19:83–9.
Feichtinger M, Reiner A, Hartmann B, Philipp T. Embryoscopy and karyotype findings of repeated miscarriages in recurrent pregnancy loss and spontaneous pregnancy loss. J Assist Reprod Genet. 2018;35:1401–6.
Hassold TJ. A cytogenetic study of repeated spontaneous abortions. Am J Hum Genet. 1980;32:723–30.
Warburton D, Kline J, Stein Z, Hutzler M, Chin A, Hassold T. Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Evidence from 273 women with two karyotyped spontaneous abortions. Am J Hum Genet. 1987;41:465–83.
Maslow BS, Budinetz T, Sueldo C, Anspach E, Engmann L, Benadiva C, et al. Single-nucleotide polymorphism-microarray ploidy analysis of paraffin-embedded products of conception in recurrent pregnancy loss evaluations. Obstet Gynecol. 2015;126:175–81.
Popescu F, Jaslow CR, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579–87.
Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod. 2002;17:446–51.
Skrzypczak J, Kwinecka-Dmitriew B, Zakrzewska M, Latos-Bielenska A. Do chromosomal abnormalities reappear in subsequent pregnancies and how often? Ginekol Pol. 2010;81:681–6.
Ulm JE. Recurrent trisomies: chance or inherited predisposition? J Genet Couns. 1999;8:109–17.
Robinson WP, McFadden DE, Stephenson MD. The origin of abnormalities in recurrent aneuploidy/polyploidy. Am J Hum Genet. 2001;69:1245–54.
Filges I, Manokhina I, Peñaherrera MS, McFadden DE, Louie K, Nosova E, et al. Recurrent triploidy due to a failure to complete maternal meiosis II: whole-exome sequencing reveals candidate variants. Mol Hum Reprod. 2015;21:339–46.
Enciso M, Sarasa J, Xanthopoulou L, Bristow S, Bowles M, Fragouli E, et al. Polymorphisms in the MTHFR gene influence embryo viability and the incidence of aneuploidy. Hum Genet. 2016;135:555–68.
Sazegary A, Kalantar SM, Pashaiefar H, Mohtaram S, Honarvar N, Feizollahi Z, et al. The T657C polymorphism on the SYCP3 gene is associated with recurrent pregnancy loss. J Assist Reprod Genet. 2014;31(10):1377–81.
McCoy RC, Demko Z, Ryan A, Banjevic M, Hill M, Sigurjonsson S, et al. Common variants spanning PLK4 are associated with mitotic-origin aneuploidy in human embryos. Hum Genet. 2015;348(6231):235–8.
Mertzanidou A, Wilton L, Cheng J, Spits C, Vanneste E, Moreau Y, et al. Microarray analysis reveals abnormal chromosomal complements in over 70% of 14 normally developing human embryos. Hum Reprod. 2013;28(1):256–64.
Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20(4):571–81.
Delhanty JD, Harper JC, Ao A, Handyside AH, Winston RM. Multicolour FISH detects frequent chromosomal mosaicism and chaotic division in normal preimplantation embryos from fertile patients. Hum Genet. 1997;99(6):755–60.
Warburton D, Dallaire L, Thangavelu M, Ross L, Levin B, Kline J. Trisomy recurrence: a reconsideration based on North American data. Am J Hum Genet. 2004;75:376–85.
Al-Asmar N, Peinado V, Vera M, Remohi J, Pellicer A, Simon C, et al. Chromosomal abnormalities in embryos from couples with a previous aneuploid miscarriage. Fertil Steril. 2012;98:145–50.
De Souza E, Halliday J, Chan A, Bower C, Morris JK. Recurrence risks for trisomies 13, 18, and 21. Am J Med Genet A. 2009;149a:2716–22.
Funding
The study was supported by the Ministry of Science and Education of the Russian Federation (budgetary project of the Research Institute of Medical Genetics, TNRMC, # AAAA-A19–11902089005-5).
Author information
Authors and Affiliations
Contributions
NTV designed the study, performed sample collection and cell culture, conducted data analysis and interpretation, and drafted the manuscript. SEA and SNN performed cytogenetic analysis of G-banded metaphase chromosomes. ZDI contributed to CGH analysis, and TEN performed cell culture. LIN contributed to sample collection and analysis and revised the article for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Scientific Ethics Committee of the Research Institute of Medical Genetics of the Tomsk NRMC and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikitina, T.V., Sazhenova, E.A., Zhigalina, D.I. et al. Karyotype evaluation of repeated abortions in primary and secondary recurrent pregnancy loss. J Assist Reprod Genet 37, 517–525 (2020). https://doi.org/10.1007/s10815-020-01703-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01703-y